Main
Cascade Metrix [CMX] in partnership with Purdue University’s Pharmacology Testing Facility (West Lafayette, IN) conducted a 3-day pilot study on two pigs. The study purpose was to inform the design and assess performance of the ex-vivo automated glucose monitoring system (AGMS) prototype developed by CMX (see Figure 1). At user defined measurement intervals, the device withdraws blood from the subject’s vascular line for measurement across a flow-through glucose sensor followed by complete reinfusion of the blood. Before each measurement the system performs an automated calibration. The study was conducted from March 21-24, 2016.

Fig 1: AGMS Prototype
Trial Set-Up: Two young pigs [2 months old, each weighing 30 kg] were tethered to two devices placed adjacent to movement-responsive caging stations (Figure 1) from BASI (West Lafayette, IN).

Figure 2: Trial Set-up at the Purdue Pharmacology Testing Facility
Central venous catheters were surgically placed in the jugular veins of the pigs. Each pig was tethered to a device using a 10 feet long micro-bore tubing. There were 2 devices [Dev1 and Dev2]. For Dev1, the flow-through glucose sensor was placed proximal to the subject secured to the tubing, while for Dev2, the flow-through sensor was placed proximal to the device enclosure. There were 2 fluid bags filled with non-heparinized buffered saline. One bag was used for rinsing, while the other was for calibration [100 mg/dl reference]. Both the devices were controlled from a single Tablet PC. The study period was 72 hours.
Results: Dev1 was able to perform the automated sampling routine for the entire 3 day period. Dev2 on the other hand encountered sluggish blood flow on Day 2, likely due to the long blood flow path of 10 ft. for each measurement cycle. The sluggish flow was resolved through a manual flush (thereby opening up the thrombus sheath) and by bring the flow-through sensor proximal to the subject (thereby reducing the blood volume required to be drawn out of the subject).
On day 3, an intravenous GTT (Glucose Tolerance Test) study was done on the pigs. 30ml of D50W was infused into the pig’s central line and multiple reference measurement were performed during the study [Figure 3].
Figure 3: Infusion of Dextrose for the Glucose Tolerance Study
The results from the GTT study are shown in figure 4 and figure 5 below. The glucose as measured by the device is shown in BLUE, while the reference glucose measurements are marked in RED.
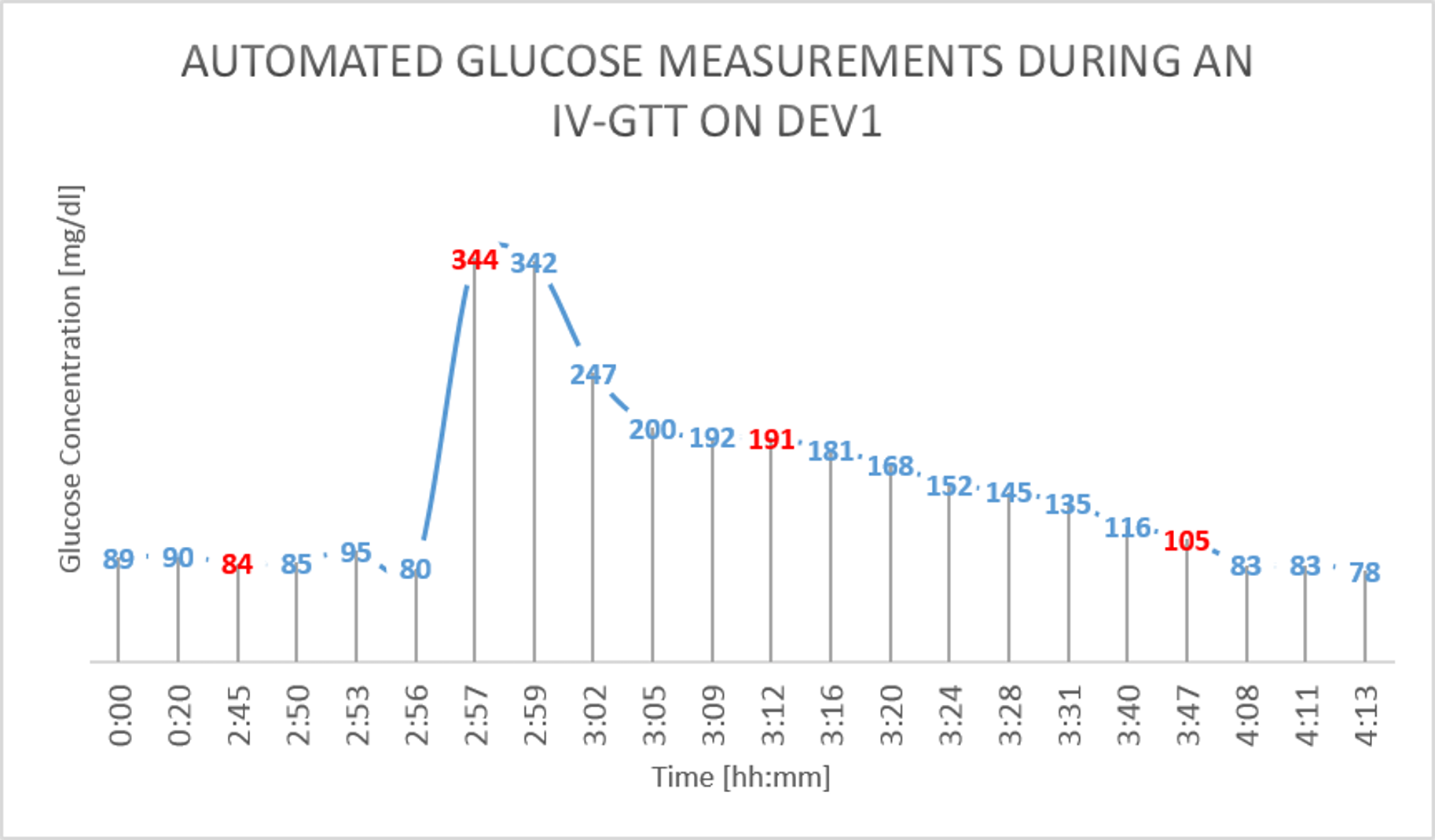
Figure 4: IV Glucose Tolerance Study on Dev1
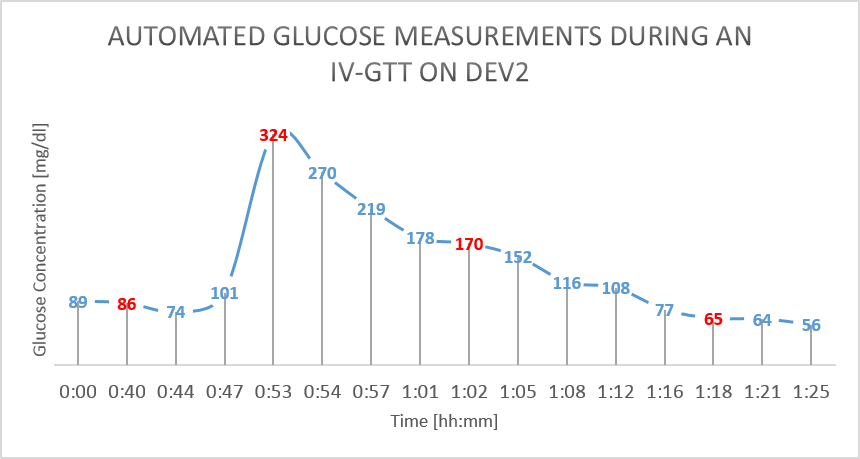
Figure 5: IV Glucose Tolerance Study on Dev2
Conclusion and Next Steps: The study demonstrated the reliability of automated sampling and glucose sensing in tethered pigs over a 3-day period without the need for heparin infusion. It is imperative that the blood volume drawn out of the subject is minimized by securing the flow-through sensor as close to the subject as possible. This will minimize the risk of thrombus sheath formation at the catheter tip. Additional studies on small animals are indicated after implementing the device design changes identified from this study, including expanding the range of measured analytes.
Need for Inpatient Glycemic Control
The intensive care units have a very high prevalence of hyperglycemia (> 40%). Additionally, hypoglycemia and glycemic variability are well recognized risk factors. It was not until 2001 that a “landmark” study by Berghe et.al. established the need for tight glycemic control [i.e., normalizing glucose through intensive insulin therapy] in ICUs. Significant reduction in mortality, morbidity (due to sepsis, renal failure, blood transfusion), and length of stay (LoS) savings were observed. Financial savings were estimated at $3,345/patient due to reduced LoS and avoided interventions. However despite protocols calling for tight glycemic control, there was poor compliance due to the existing labor-intensive and sub-par accuracy measurement methods which were typically based on capillary fingerstick sampling and meter/strips. The US Medicare system instituted “no-pay” guidelines for complications that arise from poor glucose control.
Challenges with Sepsis Management
Sepsis (defined as a dysregulated host response to infection that causes life-threatening organ dysfunction) is typically caused by blood-borne infections, which may be bacterial, viral or otherwise. Specifically, upon the earliest suspicion of Sepsis, the blood lactate levels should be assessed as part of the Surviving Sepsis Protocol, which helps to rule out or identify the root cause of a lactate elevation. If lactate is elevated (> 2 mmol/L), it can be due to elevated glucose values (Glucose monitoring would address this), internal bleeding (Hemoglobin would address this), need for resuscitating fluids (Central Venous Oxygen Saturation, ScvO2 would address this), organ failure (rare, but heart monitor can address), and blood infection. The knowledge of the "big four" analytes enables the "race" to administer triple anti-biotic therapy, which is today delayed considerably.
Existing Solutions
Currently, blood glucose monitoring is done using labor intensive fingerstick based meters/strips offered by companies like Roche Diagnostics, Nova Biomedical, and Abbott. Multi-analyte measurements are done using products like the i-Stat which uses single use cartridges or blood gas analyzers based on multi-use cartridges from companies like Radiometer (Danaher), Instrumentation Laboratories and Siemens Healthineers. Not only are blood gas analyzers labor intensive, they also are associated with long turn around times, have high cost, expose the care giver to infectious blood and above all result in substantial blood loss to the patient. Central venous oxygen saturation measurements utilize dedicated central venous catheter based fiber-optic systems from companies like Edwards Lifesciences and ICU Medical, but their use remains restricted.
Thus the CMX 4-plex solution provides a one of its kind system for early glycemic management and early Sepsis diagnosis and treatment that is associated with significant mortality and morbidity reductions.
Cascade Metrix has innovated upon an ex-vivo automated blood sampling system and multiplex analytical platform ("AutoPlexer") that is designed to enable increased adoption of multiple POC medical diagnostic applications currently constrained by workflow challenges stemming from manual blood sampling methods. The AutoPlexer enables automated, multi-analyte, closed loop cost-effective measurements for effective Sepsis and Glycemic Management. The company's multiplex patient attached solution enables accurate delivery of lactate, glucose, hemoglobin and oxygen saturation blood measurements without the need for caregiver sample handling leading to infection control and medical error issues. Additionally, the CMX system is automated and closed loop which does not create any blood waste. This is a major advantage as long-stay ICU patients (which represent 80% of the bed days, but only 50% of the patients) become anemic owing to blood testing alone. Anemia itself has been associated with death in the ICU.
The AutoPlexer automatically samples micro-liter quantities of whole blood from a patient and performs frequent measurements of various blood constituents without wasting a single drop of blood. The CM system introduces a whole new paradigm in patient monitoring that will both optimize nursing efficiency and improve patient care.
Other Blood Analytes: CMX plans to add other time sensitive tests to the AutoSampler platform. Whole blood sensors to monitor heparin therapy, blood gases, electrolytes and certain biomarkers will be integrated to the CM sampling platform. Combining leading edge innovations in micro-fluidics, electro-chemistry, and machine intelligence, CMX has developed patentable inventions covering technologies, designs, and other relevant operational aspects.
Technology Development History: Over the years, Cascade Metrix has developed multiple technologies for glucose sensing and automated blood sampling. The picture below gives a snapshot of the various technologies developed and prototyped by CM, starting with mid infrared quantum cascade laser spectroscopy based glucose sensors to a version that uses electrochemical sensors. CM's transition from infrared spectroscopy to electrochemistry based sensing was described in a research article published with the Journal of Diabetes Science and Technology
A fully functional AutoPlexer device with multi-analyte capabilities (including, glucose, lactate, hematocrit, and oxygen saturation) has been developed and undergone successful pre-clinical studies. Preparation for human/clinical studies are underway that will be followed by regulatory filings.
CAUTION: Investigational device, limited by federal law to investigational use.
FOUNDERS
Frank Lloyd, Jr, M.D. MBA is the Co-founder and President of Cascade Metrix. He is leading the clinical operations of Cascade, in addition to helping raise private investments. He has led clinical research and development through his association with industry and academia for more than 20 years. He has also held appointments as a Clinical Associate Professor in Surgery at the Indiana University School of Medicine, President of the Center of Surgical Sciences and Trauma, and Medical Director of Methodist Research Institute. Dr. Lloyd graduated from IU School of Medicine, followed by a residency in general surgery from Howard University (Washington DC), and a fellowship in surgical oncology from Roswell Park Memorial Hospital (Buffalo, NY). He also completed the physician MBA program from IU's Kelley School of Business. He has been elected twice as the Coroner of Marion County, Indianapolis.
Kislaya Kunjan, PhD, MBA is the Co-founder and CEO of Cascade Metrix. He is the lead inventor of the product technology and is managing the company's product and business development aspects in collaboration with its partners and strategic investors alike. He holds an M.S. in Mechanical Engineering from Purdue (West Lafayette, IN) and an MBA from Cornell University (Ithaca, NY). While at Purdue, he designed and developed a novel infrared glucose sensing system under the mentorship of Prof. Jay Gore. His prior professional experience spans sales engineering with Saint-Gobain, medical device development with Vista Biosciences and most recently a product management role with Johnson & Johnson/LifeScan. He completed his PhD in Health Informatics from the Indiana University School of Informatics and Computing (Indianapolis, IN).
David T. Giddings is a Co-Founder and serves as Chief Business Officer (CBO) of Cascade Metrix. His business career has been focused on healthcare, information technology, and life sciences businesses with both established and emerging publicly & privately held companies. He was with Eastman Kodak in General Management positions in Kodak's commercial Imaging and Information Systems and Healthcare Groups. Mr. Giddings joined Boehringer Mannheim Corporation in 1991 as VP and General Manager of their diagnostics Division and subsequently became President and COO of the company. He was appointed Chairman, President, and CEO of Diametrics Medical in 1995, a NASDAQ traded company, where he served for 6 years. Mr. Giddings served as Digilab Inc’s President & CEO until July, 2009, and remains an active shareholder in the company.
ADVISORY BOARD
Peter Rule has had a 30 year career leading medical device and diagnostic companies from early start up to beyond an IPO. In diabetes, Peter led MiniMed as its President and Chief Operating Office and member of the Board of directors (now Medtronic Diabetes Care), establishing the insulin pump market in the US and starting the CGM franchise as a separate team. He was also a co-founder and Chairman of the Board of Therasense (now Abbott Diabetes Care), establishing the direction for the first ultra-low blood sampling meter (“Freestyle”) and the Navigator CGM. Other successful companies Peter has led include PercuSurge, the world’s first embolic protection company in interventional cardiology, also purchased by Medtronic, and Ekos Corporation, a drug device delivery company for the treatment of Pulmonary Embolism, DVT, and PAO, purchased by BTG. Peter has been involved in the design of over 20 clinical trials, and was on the advisory board of the Diabetes Control and Complications Trial (DCCT). Peter is a named inventor on over 50 US patents. Recently, Peter led the creation of the first ICU CGM at OptiScan Biomedical, leaving after gaining FDA approval. Peter currently consults to early stage and established device and diagnostic companies, through his consulting firm PRI. Clients include breakthrough diabetes companies. Peter has an undergraduate degree from the University of Southern California and an MBA from Harvard University.
Peter T. Kissinger, Ph.D., is the founder of Bioanalytical Systems, Inc., (NASDAQ BASI) - a life science contract research firm, which he led from 1974-2007, and is Professor of Bioanalytical Chemistry at Purdue University. Dr. Kissinger has published more than 230 scientific papers and is a Fellow of the American Association of Pharmaceutical Scientists and the American Association for the Advancement of Science. In 2005, he became the Chairman of Prosolia, which markets mass spectrometry innovations for life science, industrial and homeland security applications. In 2007, he and Candice Kissinger founded Phlebotics, Inc., a medical device company focused on diagnostic information for intensive care medicine.
